How Many Electrons Are in the Outer Shell of Aluminum
5291 College Avenue in Oakland CA 94618. The first 10 electrons are in the filled first and second shells.
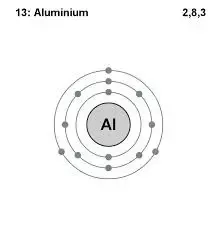
How Many Valence Electrons Are In Aluminium Quora
6 How many shells are there in chlorine atom.

. It would tend to lose three electrons and form a. 7 Does phosphorus have 4 shells. 2 How many electron shells are there.
10 Where are electrons located in an atom. The electron configuration for Aluminum is. 12 How are electrons arranged in an atom.
Keeping this in view how many energy levels does aluminum have. How many electrons does aluminum have in its outer shell. The nex six electrons will go in the.
Looking at the picture you can see there are two electrons in shell one eight in shell two and three in shell three. So for the element of ALUMINUM you already know that the atomic number tells you the number of electrons. It would tend to gain one electron and form a -1 ion.
Thus the valence electrons aluminum have for bonding is 3. Since 1s can only hold two electrons the next 2 electrons for aluminium go in the 2s orbital. Furthermore how many electrons will Aluminum gain or lose.
That means there are 13 electrons in a aluminum atom. 5 HaveThe boron atom has only six electrons in its outer shell leading to an electron deficiency. 11 What is the system of electrons surrounding the nucleus of an atom.
Thus the valence electrons aluminum have for bonding is 3. 5 How many total shells can an atom have. How many protons are in indium.
Aluminum is a metal which have 3 valence electrons as there are 3 electrons in the outermost shell of the element. 9 How do you find the outer shell electrons. This molecule has 12 valence shell electrons.
3 each from the B atoms and 1 each from the six H atomsAug 21 2020How many valence electrons. 7 Which type of elements have their outermost shell incomplete. When we write the configuration well put all 13 electrons in orbitals around the nucleus of the Aluminium atom.
Aluminum electron configuration That is the first shell of aluminum has two electrons the second shell has eight electrons and the 3rd shell has three electrons. 4 How many shells does the P Subshell have. Aluminum is in the fifth column and therefore has 5 electrons in its outermost shell.
Aluminum contains 3 electrons in its outer shell thus it has 3 valence electrons. This leaves only three electrons in the third shell 3s23p1. Looking at the picture you can see there are two electrons in shell one eight in shell two and three in shell three.
8 How many shells will be there in an atom having 15 protons. How many electrons are in the outer shell of aluminium. Aluminum is a metal which have 3 valence electrons as there are 3 electrons in the outermost shell of the element.
That means there are 13 electrons in a aluminum atom. 3 How many electrons are there on the outer shell of phosphorus. How many electrons does aluminum have in its outer shell.
13 10 3. Aluminum gallium indium and thallium have three electrons in their outermost shell a full s orbital and one electron in the p orbital with the valence electron configuration ns 2 np 1. 13 How many electrons are there in.
So there are only 3 valance electrons. 8 Which elements had a filled outermost shell. In writing the electron configuration for Aluminium the first two electrons will go in the 1s orbital.
How Many Valence Electrons Does Boron b Atomic No. Alden cordovan shoes for sale. 5 rows The first shell of Aluminum has 2 electrons and the outer shell or valence shell of.
The electron configuration of the aluminum shows that there are two electrons in the K shell eight in the L shell and three in the M shellorbit. The electronic configuration of Antimony is 2 8 3. Electrons is the number on the top rightcorner with the.

How Many Valence Electrons Does Aluminum Al Have
Comments
Post a Comment